Pipeline
Our Pipeline
The drug candidate GT-002 is a fast-acting selective high-affinity GABAA receptor modulator with an attractive profile. It is safe and well-tolerated in healthy volunteers with a favorable pharmacokinetic profile. Based on preclinical and clinical data, further clinical development of GT-002 as a therapeutic for psychiatric disorders is ongoing, especially in disorders with an associated cognitive dysfunction.
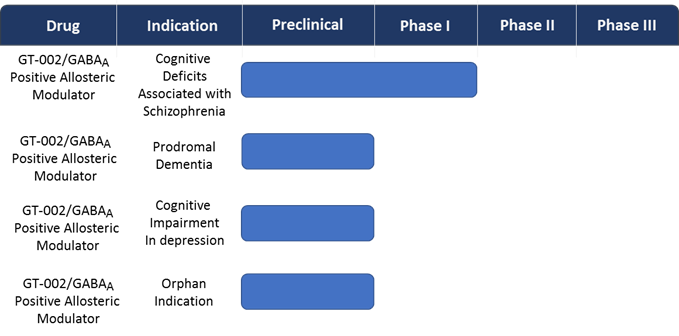

Gabather’s current clinical pipeline contains 3 different potential indications with cognitive impairment as the common denominator. The pro-cognitive effects of GT-002 and the potential therapeutic use of GT-002 in these neuropsychiatric disorders are under investigation. In addition, the potential use of GT-002 for an orphan indication is also under investigation and based on the outcome from these studies an orphan drug application will be submitted.GT-002 has completed the phase I program in healthy volunteers and is phase II ready. Before initiating patient studies an EEG/fMRI target engagement study in healthy volunteers will be conducted.
Read more:
Alzheimer´s Disease
https://www.alz.org/alzheimer_s_dementia
https://en.wikipedia.org/wiki/Alzheimer%27s_disease
Major Depressive Disorder
https://www.nimh.nih.gov/health/topics/depression
Orphan drug
https://www.ema.europa.eu/en/human-regulatory/overview/orphan-designation-overview